Abstract
Background: Venous thromboembolism (VTE) is a known complication of cancer, with a high incidence in patients with both gliomas and lymphoma. Recent studies have shown a high risk of intracranial bleeding in glioma patients treated for VTE with anticoagulation. To date, there are no large, population-based studies describing the incidence of VTE in patients with primary central nervous system lymphoma (PCNSL).
Methods: Using the California Cancer Registry, we identified patients with a first histologic diagnosis of PCNSL from 2005-2014 and linked these cases to the California hospitalization and emergency department databases. Patients with a VTE within 6 months prior to PCNSL diagnosis were excluded (n=11). We calculated cumulative incidence of VTE and major bleeding and associated 95% confidence intervals (CI), adjusted for the competing risk of death. Multivariable Cox proportional hazards regression models, using the methods of Fine and Gray to adjust for competing risk of death, were used to analyze factors associated with VTE and major bleeding. Models included sex, race/ethnicity, age at diagnosis, neighborhood sociodemographic status, health insurance at diagnosis, Elixhauser comorbidities, HIV status, initial treatment (chemotherapy, radiation, or CNS procedure), and prior VTE (> 6 months prior to diagnosis). The major bleeding model additionally included VTE type as a time dependent covariate. The association of VTE and major bleeding with PCNSL-specific mortality was analyzed using multivariable Cox proportional hazards regression models; VTE and major bleeding were included as time dependent covariates. Results are presented as adjusted hazard ratios (HR) and 95% CI.
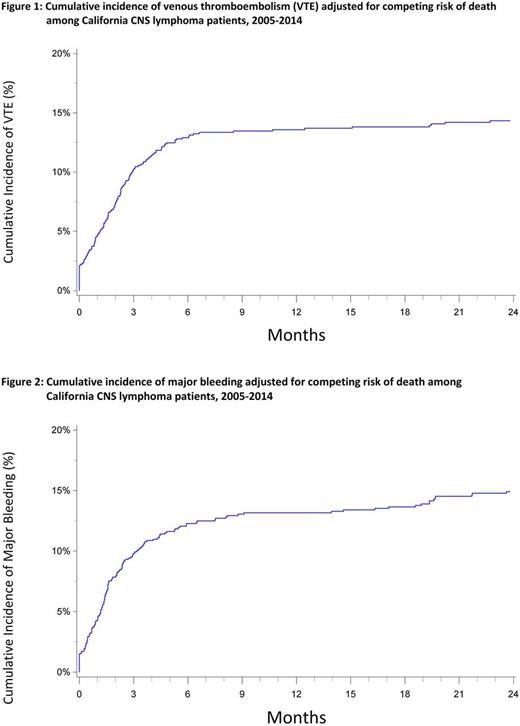
Results: There were 992 patients with a PCNSL identified. VTE occurred in 143 patients (14.4%). Of the VTE events, 52% were pulmonary emboli [(PE +/- deep vein thrombosis (DVT)], 23% proximal DVT and 22% distal DVT. The 3- and 12-month cumulative incidences of VTE were 10.2% (CI: 8.4-12.2%) and 13.6% (CI: 11.5-15.8%), respectively (Figure 1). Patients who received chemotherapy had over 2-fold increased risk of developing VTE (HR=2.42, CI: 1.33-4.42) compared to those who did not receive chemotherapy, and those who received radiation were also at increased risk of VTE (HR=1.56 CI: 1.07-2.27). Asian/Pacific Islanders had a decreased risk of VTE compared to non-Hispanic Whites (HR=0.37, CI: 0.21-0.66).
Major bleeding occurred in 156 patients (15.7%). Of the major bleeding events, 53% were intracranial hemorrhage, 33% were gastrointestinal bleeds, 12% of patients required a transfusion and 3% had unspecified bleeding. The 3- and 12-month cumulative incidences of major bleeding were 9.8% (CI: 8.1-11.8%) and 13.2% (CI: 11.1-15.3%), respectively (Figure 2). PE and proximal DVT were associated with increased risk of major bleeding (HR=4.57, CI: 2.43-8.60 and HR=5.95, CI: 2.47-14.34, respectively).
In the PCNSL specific mortality models, PE was associated with increased risk of death (HR=1.81, CI: 1.14-2.87), though DVT (proximal or distal) was not. Patients with major bleeding were at over 2-fold increased risk of PCNSL death compared to those without major bleeding (HR=2.34, CI: 1.71-3.19).
Conclusions: The incidence of VTE in this large population-based study of patients with PCNSL was high at 14.4%, with most VTE events occurring within the first 3 months after diagnosis. Risk factors associated with VTE included treatment with either chemotherapy or radiation. PE and proximal DVT were associated with increased risk of major bleeding, suggesting these patients may have received anticoagulation, and as recently shown in glioma patients, are at a high risk of intracranial hemorrhage. In addition, PE and major bleeding were both independently associated with higher PCNSL mortality.
Wun: Janssen: Other: Study steering committee and research support (site PI); Pfizer: Other: Study steering committee and research support (site PI).
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal